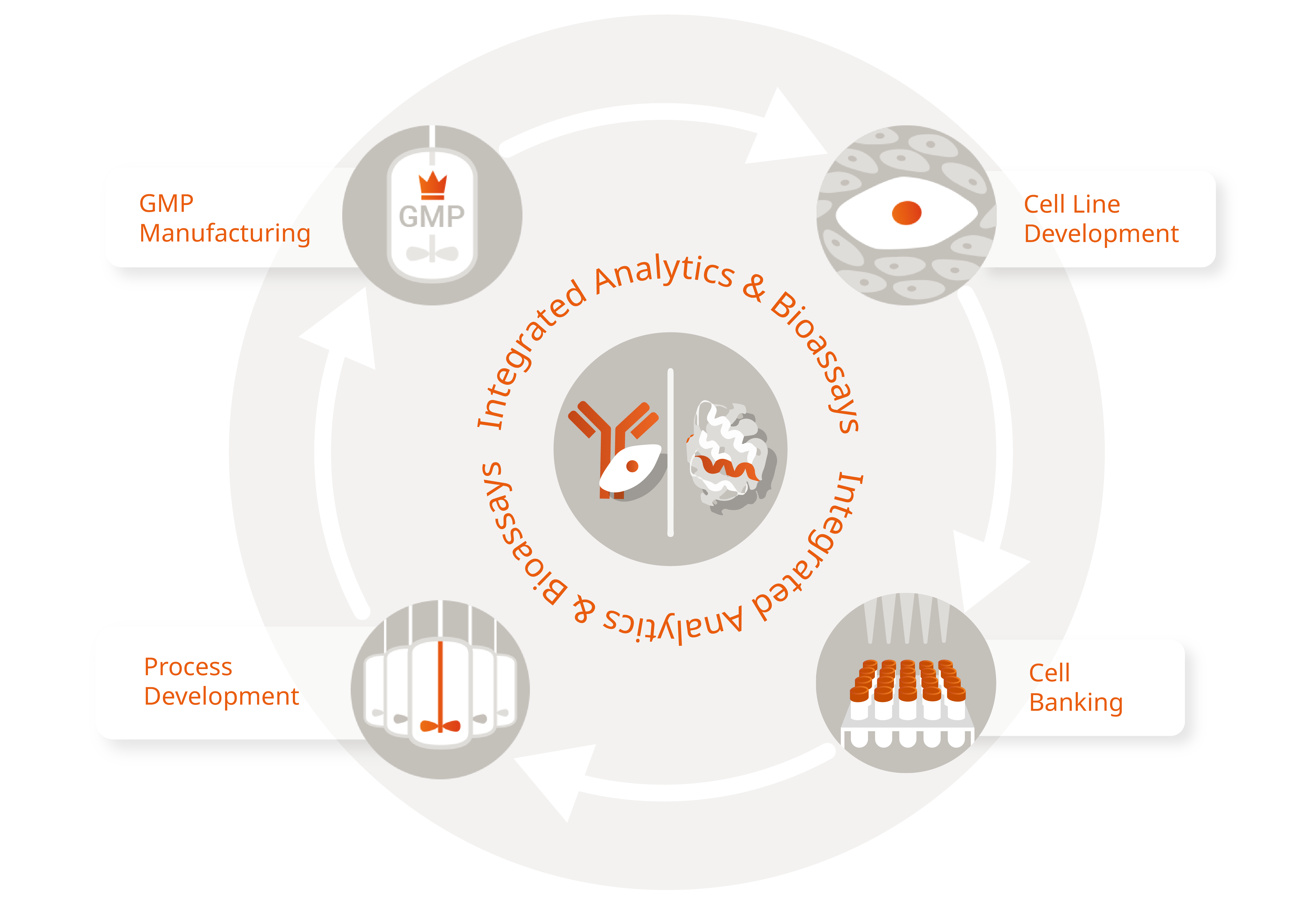
Integrated CDMO Services for Proteins and Antibodies
You have identified your lead candidates and want to transition from the preclinical to clinical stage as efficiently as possible, while ensuring quality and functionality?
Then partnering with an experienced CDMO that can provide everything from cell line and process development to (GMP) manufacturing - including comprehensive analytical services - is the best way forward!
The Experienced CDMO for Your Biologics
Whether you want to produce a conventional monoclonal, mAb fragments, a more complex multivalent mAb-like format, fusion proteins, or a therapeutic enzyme, we are your trusted and highly experienced partner for development and manufacturing. From unique cell lines to large scale production, our process is tailored to your individual requirements. Guided by our experienced project managers, we ensure a positive customer journey by navigating the entire process with expertise and care.
Expect best-in-class efficiency and competitive timelines combined with excellent customer service.
+
Cell Lines Developed
<
Months from Gene to DP
~
GMP Batches/Year
Innovative & Complex Molecules Are Our Business
Monoclonal
Antibodies
Bispecific
Antibodies
Multispecific
Antibodies
Fc Focused
Protein
Antibody
Fragments
Recombinant
Proteins
Cytokines
Enzymes
Clotting Factors
Over the years, ProBioGen has created hundreds of producer cell lines for our clients across the biopharma world. Many of these cell lines were taken to the clinic and more are now in late stage development or are being used for commercial production in the US, EU, and other locations. Modalities range from normal antibodies, various bi/multi-specific formats, and highly complex single-chain molecules to challenging biosimilars.
We produce master cell banks (MCBs) as well as working cell banks (WCBs). We start by checking for clone-specific cell growth and resistance to DMSO during the filling process. Based on the collected data, a GMP-compliant manufacturing procedure and a master batch record are written. In order to protect the interests of the customer, a QAA (quality assurance agreement) is drawn up that describes the responsibilities and the qualitative framework.
We initiate process development at an early stage in order to set the course for successful development and identify potential. Therefor we rely on material production from pools to perform the initial parameter screening. This is done in close collaboration with you in order to define process expectations.
Our integrated approach to cell line and process development minimizes unwanted variants from the start and maximizes production yield. Through inter-departmental collaboration, we optimize key process conditions to establish a robust, scalable process for consistent large-scale manufacturing.
Our expert team's long-standing experience ensures the fast and reliable conversion of all development protocols for the GMP-stage as well as the safe manufacturing of single or multiple batches. We offer our services both in-house and for externally developed processes.
We offer GMP production in fully state-of-the-art disposable systems in fed-batch and continuous processes in up to 1000L bioreactors. We rely on our own chemically-defined media platform, including in-house media and buffer preparation.
With over 30 years of experience, we possess in-depth knowledge of our processes. This enables us to offer comprehensive technology (tech) transfer solutions tailored to your needs. Transfer activities include the definition of a transfer package plan, document transfer, web-based meetings, online support, and troubleshooting as well as on-site training. Finally, the manufacturing processes are designed to meet state-of-the-art manufacturing processes, and this makes tech transfers even smoother.
Our flexible approach allows us to add or transfer projects at any time, all while ensuring that you receive the support you need exactly when you need it. Our processes are also oriented towards transferability.
CMC Navigator ™ – A Structured Framework to Select the Right CMC Path
As biologics programs advance, Chemistry, Manufacturing and Controls (CMC) requirements evolve. Different development priorities call for different CMC setups. Whether the focus is rapid first-in-human entry, preparation for later-stage transfer, targeted CMC support, or improvement of an existing cell line and the production process - clients need adaptable solutions that meet them wherever they are in their journey
CMC Navigator™ is ProBioGen’s structured framework of four clearly defined CMC service models, designed to support biopharmaceutical companies across all stages of protein CMC development. CMC Navigator provides clear entry points and transparent scope, helping teams align CMC strategy, timelines, budgets and execution with their current program priorities, so that they can move forward with clarity and control.
From DNA to clinic, on a fast track.
Sprint provides a streamlined pathway for when reaching first-in-human quickly is critical. Using integrated, platform-based workflows and a clearly defined scope, your program progresses efficiently with reduced timelines and focused execution, from sequence to GMP drug substance for clinical supply.
Built for today, ready for tomorrow.
Scale is suited for programs that require clinical material now while preparing for later-stage development or technology transfer. Your future-proof CMC setup is designed with scalability, documentation quality, and transfer-readiness considered from the outset.Only what you are ready for.
Customize applies when specific CMC activities need to be addressed within an existing development strategy. Our modular format gives you the flexibility to select only the CMC elements as stand-alone work packages – without committing to an integrated end-to-end model.
Don’t start over, start better.
Rise is intended for programs already in the clinic where CMC optimization is required. Existing cell lines or manufacturing processes are re-engineered to improve titer, robustness, performance and suitability for continued clinical development.
CMC Navigator™ - Protein CMC Collaboration Framework
Four paths. One clear way forward.
As biologics programs advance, Chemistry, Manufacturing and Controls (CMC) requirements evolve. Different development priorities call for different CMC setups. Whether the focus is rapid first-in-human entry, preparation for later-stage transfer, targeted CMC support, or improvement of an existing cell line and the production process — clients need adaptable solutions that meet them wherever they are in their journey.
CMC Navigator™ is ProBioGen’s structured framework of four clearly defined CMC service models, designed to support biopharmaceutical companies across all stages of protein CMC development. CMC Navigator provides clear entry points and transparent scope, helping teams align CMC strategy, timelines, budgets and execution with their current program priorities, so that they can move forward with clarity and control.
Sprint
From DNA to Clinic, on a Fast Track.
Sprint provides a streamlined path when reaching first-in-human is critical. With proven platform processes and clear scope, your program moves fast and efficiently from sequence to GMP drug substance.
Scale
Built for Today, Ready for Tomorrow.
Scale is suited for programs needing clinical material now while preparing for later-stage development or technology transfer. Your CMC setup is built for scalability, documentation quality and transfer-readiness.
Customize
Only What You Are Ready For.
Customize applies when specific CMC activities need to be addressed within an existing development strategy. Our modular format gives you the flexibility to select the CMC elements as stand-alone work packages.
Rise
Don’t Start Over, Start Better.
Rise supports clinical-stage programs needing CMC optimization. Existing cell lines or processes are re-engineered to improve titer, robustness and performance for continued development.
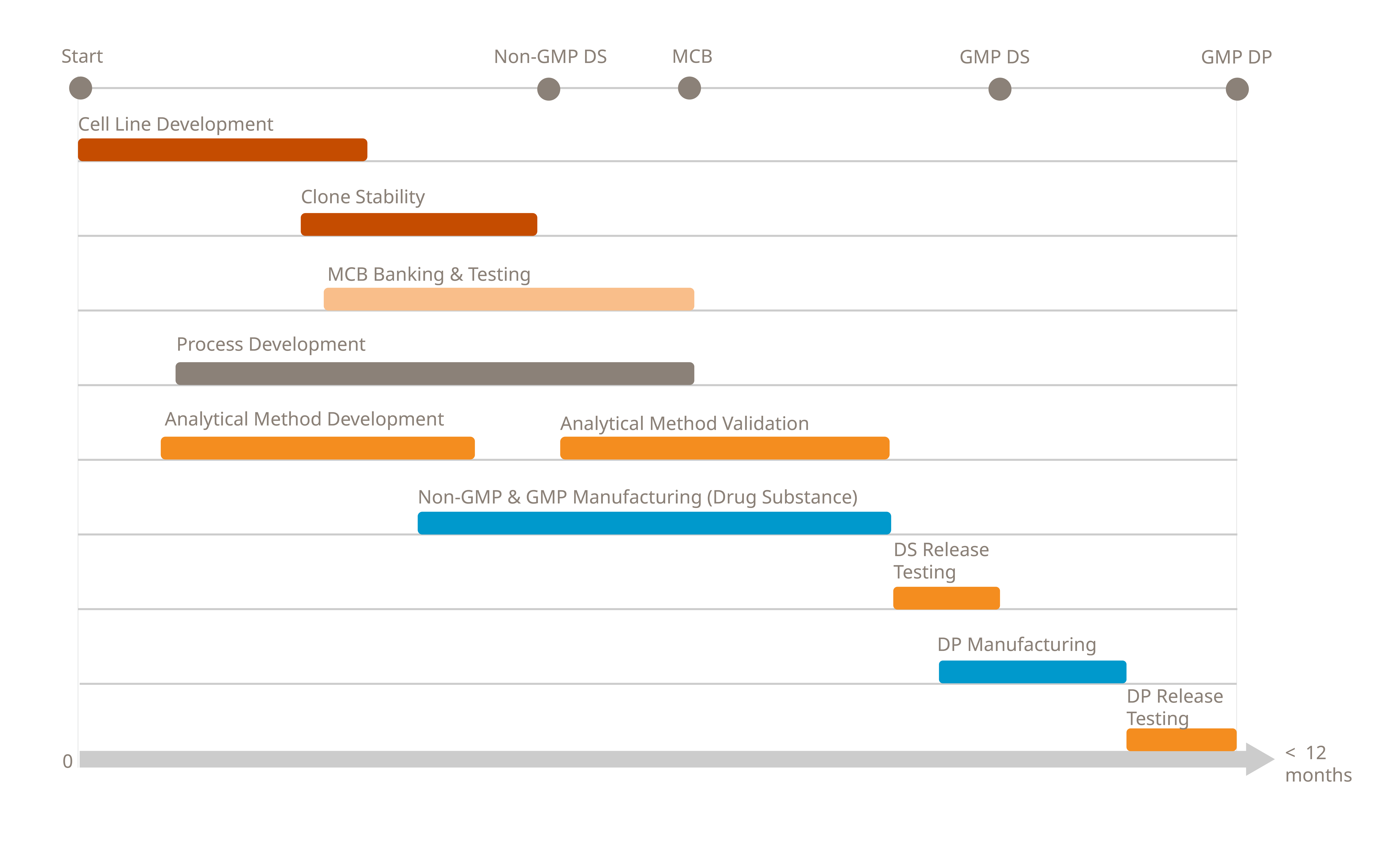
Accelerated Timelines
Achieve accelerated timelines with our integrated CDMO services, taking your antibody from sequence to IND in just 12 months*. By combining cell line development, process development manufacturing and analytics under one roof, we streamline workflows and enable overlapping project phases. This allows to drastically reduce timelines without increasing risk.
Our expert project management office (PMO) acts as a dedicated customer interface, facilitating fast decision-making, solving challenges efficiently, and driving molecules seamlessly through the process.
Our Promise To You
Rely on Fast Timelines
- One-Stop-Shop with seamless, integrated Services
- Parallel Processes
- Efficient project execution
Get Your High Quality Candidate
- Successful track record with complex molecules and projects
- Proprietary technologies elevate product quality
- Strong analytics teams and stellar quality management services
Have the Flexibility You Need
- Choose from modular offerings
- Strong partnership approach
- Experienced project managers and efficient decision-making processes
Elevate Your Candidate With The Right CDMO
Explore Related Services
Explore Related Content

Blog Post








