AAV Production
Whether you need a high quality AAV vector for preclinical research or for clinical trials, we can support you with a vector production service to match your needs. As a service provider of viral products, we offer vector design, process development, and manufacturing for research purposes and clinical batch—flexible, efficient, and compliant.
AAV Development and Manufacturing for Gene Therapy and Research
At ProBioGen, we are dedicated to advancing gene therapy and research through the development and production of high-quality AAV (adeno-associated virus) vectors. Our expertise, our cutting-edge technology, and our commitment to quality make us your ideal partner for your AAV vector production.
We offer flexible, scalable solutions designed to meet the specific requirements of your project, from research and development to clinical trials and therapeutic applications.
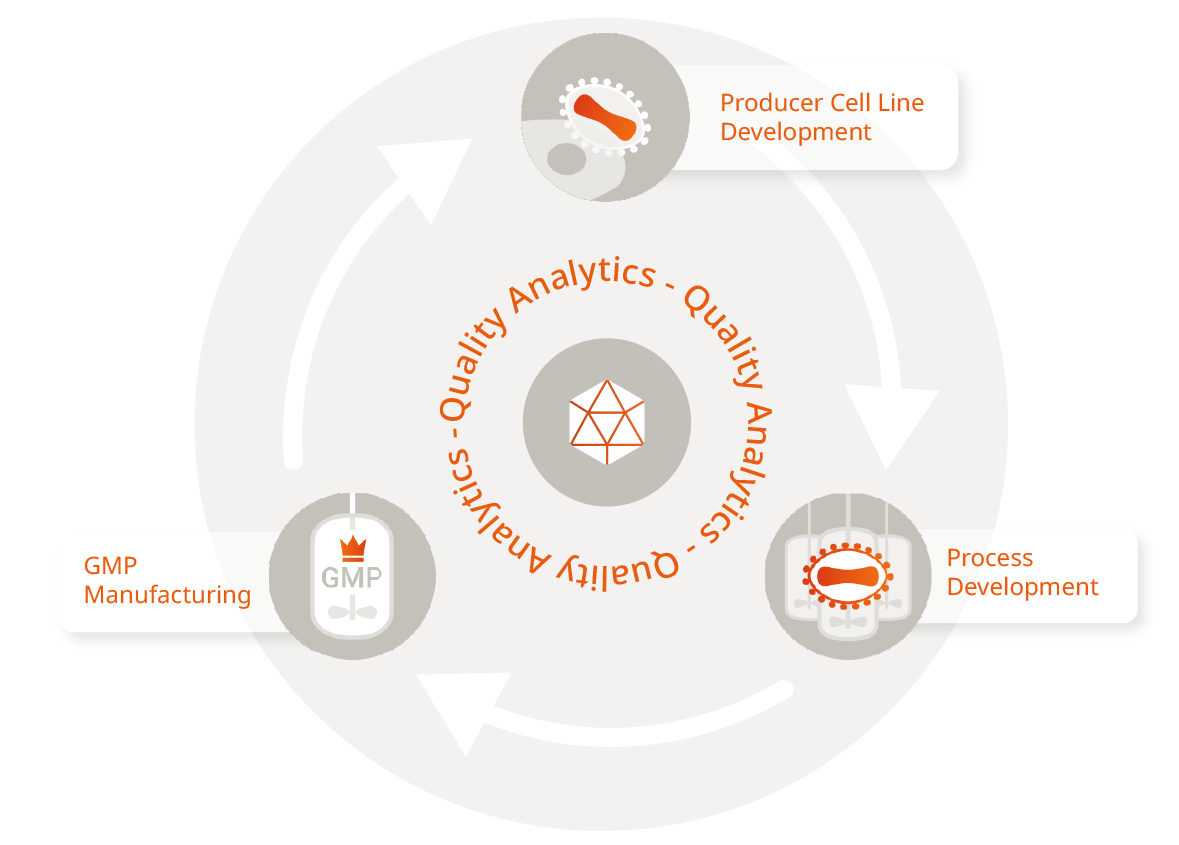
Integrated Services on Demand
We offer flexible production platforms for high-yield AAV vectors, which enables us to meet the diverse needs of your preclinical and clinical development program. By genetically optimizing the transfer vector, we can tailor the expression of the gene of interest (GOI) to your needs and your individual therapeutic application. We also offer a wide variety of AAV serotypes, which allows you to choose the most suitable vector for your research or therapeutic goals. Our current capabilities include the production of standard and engineered serotypes, including AAV2 and AAV9. We work closely with our clients to select the optimal serotype, thereby ensuring efficient tissue targeting and therapeutic efficacy.
-
Upstream Process (USP) Development
Our USP team, with a deep understanding of process dynamics, focuses on optimizing the production process to ensure scalability and reproducibility and to achieve high-yield AAV vectors. We optimize conditions for both transient transfection and packaging cell line platforms using our proprietary HEK293 cell line to guarantee robust production and flexibility to accommodate diverse project requirements.
-
Downstream Process (DSP) Development
After successful AAV vector production, our DSP capabilities ensure the efficient purification and preparation of the vectors. Our downstream process includes cutting-edge techniques for chromatography, tangential flow filtration (TFF), and virus polishing, resulting in high-purity AAV vectors that meet stringent quality standards. We work closely with clients to customize DSP strategies that comply with specific purity, yield, and regulatory requirements.
State of the Art Production Platforms
Once the vector design has been finalized, we employ state-of-the-art production platforms and technologies to generate AAV vectors with exceptional quality and consistency. Our production facilities adhere to strict regulatory guidelines to ensure the safety and integrity of the vectors produced.
We offer both preclinical research production up to 25L as well as GMP-grade AAV production up to 200L bioreactor scale. Our GMP facility is equipped with an automated fill & finish device to guarantee aseptic filling for AAV gene therapy products.
Streamlined Tech Transfer for AAV Viral Vectors
We also offer seamless technology transfer solutions for AAV viral vectors, which are tailored to meet your large-scale manufacturing needs. Our experienced project managers lead every step of the process, ensuring a smooth transition from inception to execution. With a hands-on approach and a commitment to excellence, we deliver optimal results and a positive customer experience at any stage.
Our AAV analytics service ensures the quality, potency, and safety of your AAV vectors through comprehensive characterization and testing. We utilize a wide range of analytical methods to provide detailed insights into vector quality and performance. This guarantees that your vectors meet both scientific and regulatory requirements.
Key AAV analytics include:
- Quantitative PCR (ddPCR and qPCR): accurate measurement of vector genome copies
- Infectivity assays: measurement of functional AAV particles for potency assessments
- Capsid titer testing: quantification of the total number of viral particles by ELISA
- Purity and identity testing: analysis of host cell protein/DNA contaminants to secure the purity and identity of your AAV vector
- Full characterization reports: detailed documentation for regulatory submissions and quality assurance
Take the Right Pathway to AAV Production
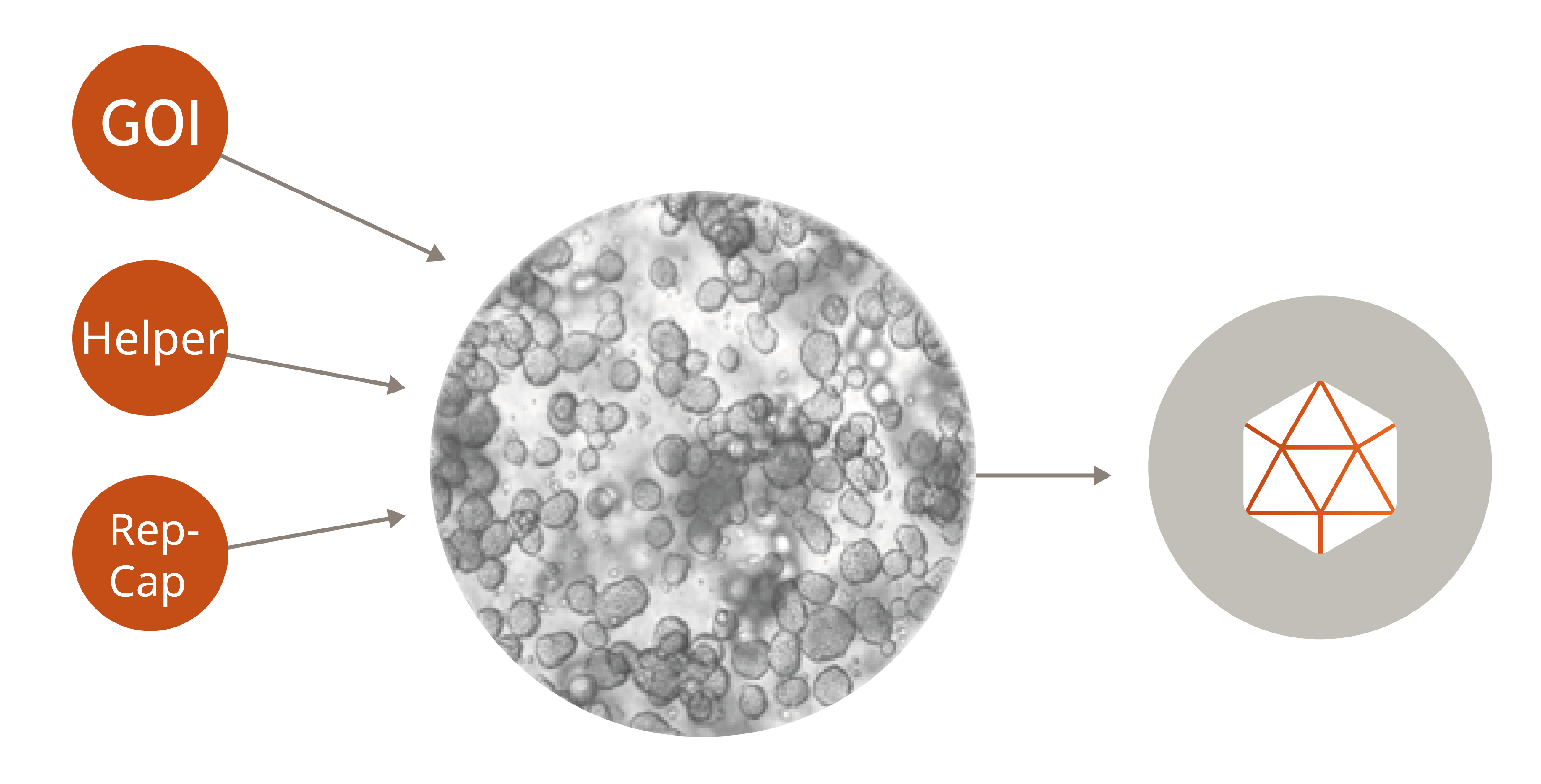
Fast Track & Small Scale: Transient Transfection
When to Adopt This Approach:
This method is widely used due to its flexibility and ability to produce AAV fast and in the short term. It is ideal for preclinical research, proof-of-concept studies, and projects that require rapid turnaround times. Our optimized transient transfection protocols ensure consistent, high-titer vector yields, which helps streamline your research and development workflows.
Production Scale: Preclinical Research Production or GMP-Grade AAV Manufacturing
For preclinical research, we can already offer scalable AAV production in bioreactors of up to 25L. This provides an efficient and cost-effective solution for early-stage projects that require higher yields than traditional small-scale production methods. We use wave, orbital shaken, and stirred tank bioreactors, depending on the customer's needs.
Our GMP-compliant facility, currently in development, will include a 200L bioreactor designed for large-scale production, supporting both clinical trials and commercial demands. This scalable solution ensures seamless project progression, from preclinical research to clinical application, while maintaining full regulatory compliance.

Stable Production at Larger Scale: Packaging Cell Line*
When to Adopt This Approach:
For scalable vector production, we will be able to offer AAV generation through established packaging cell lines in the near future. This approach is preferred for large-scale and clinical-grade projects as it offers batch-to-batch consistency and scalability, making it ideal for high-demand applications and commercial production.
Production Scale: GMP-Grade AAV Manufacturing
Our GMP-compliant facility, currently under development, will feature a 200L bioreactor for large-scale production to support clinical trials and commercial needs. With this scalable solution, your project will grow seamlessly, from preclinical research to clinical application, with full regulatory compliance.
*Coming Soon
Our Proprietary HEK 293 Cell Line
Our AAV production is powered by our proprietary HEK293 cell line, which has been optimized for growth in suspension in chemically-defined mediums. This ensures a robust, scalable, and high-efficiency production platform for AAV vectors. Our cell line is prepared as a master cell bank (MCB), thereby ensuring a consistent and high-quality source for vector production across all projects, from early-stage research to GMP-grade manufacturing.
Stable AAV Packaging Cell Line*
We will soon be able to offer a next-generation packaging cell line developed specifically for AAV production. Based on our optimized starter cell line and professional cloning methods including the proprietary DirectedLuck® transposase system, our innovative cell line is engineered to provide exceptional performance, reliability, and scalability, making it an ideal choice for researchers and developers in the biotechnology and pharmaceutical industries.
*Coming Soon
Our Promise To You
Do It Your Way
- End-to-end-solution, from development to GMP manufacturing
- Scalable production variants in accordance with customer needs, including tech transfer options
Do It Fast and Get It Stable aaaaaaaaaaaaaaaaaaaa
- Transient transfections allow for fast results and rapid turnarounds
- Packaging cell lines allow for stable production at larger scale
Meet Scientific and Regulatory Requirements
- Comprehensive testing, characterization and quality control
- Wide range of analytical methods



%20kooperieren%20im%20Berlin%20Center%20for%20Gene%20und%20Cell%20Therapies%20(BC%20GCT)-3.jpeg)

