Cell Line Development
You need the best performing, most stable production cell line - customized to your specific needs?
This is exactly what we deliver: our extensive experience in cell line development paired with our cutting-edge technologies guarantee the highest quality and on-time delivery, even for challenging molecule formats.
Cell Line Development Made by Real Experts
Over the years, ProBioGen has created hundreds of producer cell lines for our clients across the biopharma world. Many of these cell lines were taken to the clinic and more are now in late stage development or are being used for commercial production in the US, EU, and other locations. Modalities range from normal antibodies, various bi/multi-specific formats, and highly complex single-chain molecules to challenging biosimilars.

With a Superior Platform Proven in Large-Scale Manufacturing
Our cell line development services utilize our highly advanced DirectedLuck® transposase and transposon, our optimized host cells, and our own chemically-defined process medium. All this shape our unique CHO.RiGHT® platform. We use our CHO-DG44 strain, which has been selected and optimized for its fast growth, high genetic stability, and efficient metabolism. For molecules where distinctive product features (e. g. glycans) are required, we offer a wide range of pre-engineered hosts or other strains. And also: no license fees or strings attached!
In Collaborative Team Effort with Our Clients
We strongly embrace a partnership approach. This starts by listening very carefully to our clients to gain a deep understanding of the molecule and its properties and then adapting flexibly to their needs throughout the project. We anticipate risks, tackle challenges, and make adjustments in close cooperation with you.
Combining a Scientific Approach with Pragmatic Solutions
We gain a deep understanding of your molecule and use our scientific expertise to anticipate critical product features. Our fully integrated analytical and next generation sequencing teams ensure that scientists engage closely, which enables timely and accurate conclusions. Our passion for solving problems helps us arrive at pragmatic solutions in the shortest possible time. This makes us specialists when it comes to handling highly complex molecules such as multi chain formats.
With Smart Automation
Our modular automation concept offers unique flexibility. It allows an earlier analysis of critical product attributes for a larger number of clones. The output is clones with the highest quality level, higher titer, and a considerably accelerated workflow. On top of that, the system increases the throughput and enables parallel development of multiple molecule variants.
Leveraging Our Stellar Technologies
ProBioGen's DirectedLuck® transposase technology delivers the highest productivity, stability, and product quality. This best-in-class transposase is designed to target specific epigenetic marks and facilitate transgene integration at highly active genomic sites.
GlymaxX®, our outstanding afucosylation technology, also makes a real difference. Notably, it makes it possible to adjust any desired fucosylation level for the specific product.
Starting with Multiple Variants
Saving time and minimizing risk are good reasons to develop multiple molecule variants in parallel, without significantly increasing the timelines and costs. Perhaps you want to confirm manufacturability? Or maybe your last animal study is ongoing and you have not selected your final lead candidate yet? We have the experience and capacity to handle multiple molecule variants in parallel, even across the entire cell line development process. Our toolbox: a highly efficient expression system, fully-integrated product characterization, and smart automation.
Bulk Pools Ready for Manufacturing
Our transposase system delivers highly productive bulk pools. In these pools expression and product quality are extremely stable. In addition, their product quality features are predictive for clones. These bulk pools can be used for manufacturing at larger scale to produce material for development work or TOX studies. Such an approach relies on close interaction with the USP and DSP teams and greatly speeds time to clinic.
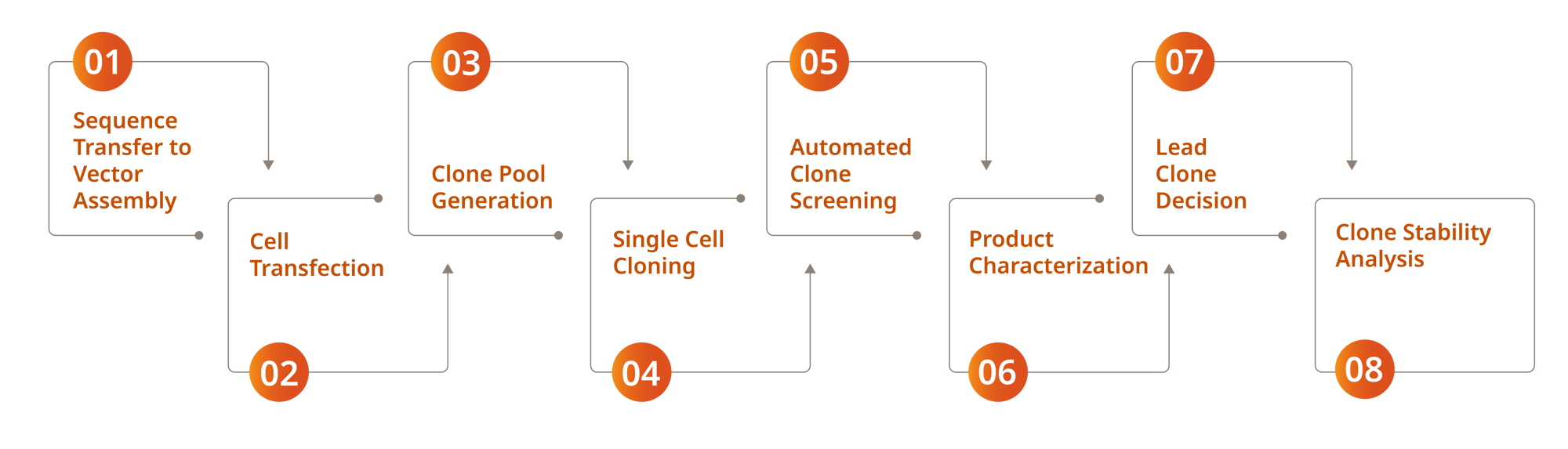
From Gene to Lead Candidate
Our Promise To You
Highest Quality
- Thanks to access to outstanding technologies
- Because of fully-integrated analytical and next generation sequencing teams
Delivered on Time
- Highly robust, yet flexible platform
- Integrated teams and processes paired with strong problem-solving skill
Ready for Manufacturing
- A fully integrated analytics team
- Deep interaction with the USP & DSP teams enables rapid transition to manufacturing
- Full traceability and regulatory compliance





